Baxalta Inc. v. Genentech, Inc., Appeal No. 22-1461 (Fed. Cir. Sept. 20, 2023)
Our Case of the Week focuses on the enablement requirement. It’s the first case to come before the Federal Circuit following the Supreme Court’s decision on the issue earlier this year in Amgen Inc. v. Sanofi, 598 U.S. 495 (2023). Here, a panel of the Circuit unanimously found that broad claims to an entire class of antibodies were not fully enabled by the patent specification.
The patent concerns treatments for hemophilia A, a blood-clotting disorder. Blood clots are formed through a series of enzymatic activations, including a step wherein Factor VIII and Factor IX/Factor IXa complex to activate Factor X. For patients with hemophilia A, Factor VIII is functionally absent.
Baxalta’s ’590 patent discloses a treatment for hemophilia A, wherein certain antibodies allow Factor IXa to activate Factor X in the absence of Factor VIII. Independent claim 1 recites:
-
-
- An isolated antibody or antibody fragment thereof that binds Factor IX or Factor IXa and increases the procoagulant activity of Factor IXa.
-
Baxalta’s inventors had generated antibodies that met the claim using traditional techniques. The inventors discovered that only 1.6% of the thousands of screened antibodies increased the procoagulant activity of Factor IXa. The patent discloses amino acid sequences of eleven antibodies that worked successfully. Each of the disclosed antibodies was either monoclonal or monospecific. The specification further disclosed that skilled artisans may use well-known antibody engineering techniques to identify other such antibodies, including bispecific antibodies and “humanized antibodies.”
The district court found the claim invalid for lack of enablement.
Under the Supreme Court’s decision in Amgen, which issued after the district court’s opinion, “the specification must enable the full scope of the invention as defined by its claims,” allowing for “a reasonable amount of experimentation.” Our write-up of the Amgen case can be found here. That means “the specification of a patent must teach those skilled in the art how to make and use the full scope of the claimed invention without undue experimentation.”
Baxalta argued the district court erred because artisans can make and identify new claimed antibodies using routine processes disclosed in the patent. The Federal Circuit did not agree, and found the facts “materially indistinguishable from those in Amgen,” which also concerned antibodies. The Court stressed that there are “millions of potential candidate antibodies, . . . but the written description discloses the amino acid sequences for only eleven.” “Just like the roadmap rejected by the Supreme Court in Amgen, the ’590 patent’s roadmap simply directs skilled artisans to engage in the same iterative, trial-and-error process the inventors followed to discover the eleven antibodies they elected to disclose.”
Additionally, the ’590 patent did not contain any disclosures like a quality common to every functional embodiment that would allow a skilled artisan to reasonably predict which antibodies will perform the claimed functions.
Accordingly, the Court affirmed the district court’s summary judgment ruling of invalidity. It should be noted this was the second appeal in this case. We wrote up the prior appellate decision as our case of the week here.
The opinion can be found here.
By Nika Aldrich
ALSO THIS WEEK
Elekta Ltd. v. ZAP Surgical Sys., Inc., Appeal No. 2021-1985 (Fed. Cir. Sept. 21, 2023)
The Federal Circuit affirmed an inter partes review decision of the Patent Trial and Appeal Board that found challenged claims of Elekta’s U.S. Patent No. 7,295,648 to be obvious. The Court held that in its findings concerning the prior art, the Board was not required to make an express “reasonable expectation of success” analysis separately from its “motivation to combine” analysis. The ’648 patent claimed a method and apparatus for delivering therapeutic radiation from a linear accelerator (“linac”) mounted on a rotating concentric ring structure:
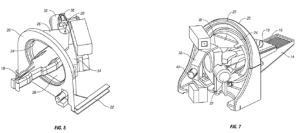
At issue on appeal was whether a person of ordinary skill in the art would have been motivated to combine a prior art reference that disclosed a similar rotational apparatus for use in radiation imaging applications (such as X-ray) with a reference generally disclosing the use of a rotating linac to deliver radiation therapy. The Board found that a POSITA would be motivated to make the combination, for example rejecting petitioner ZAP’s arguments that the added weight of the linac and associated accuracy concerns would dissuade a POSITA from making the attempt, and noting that the prior art disclosed advantages to combining imaging and therapeutic irradiating functions in a single device. The Federal Circuit agreed, and also rejected ZAP’s argument that the Board failed to make any express “reasonable expectation of success” analysis. Rather, the Court re-affirmed precedent that “a finding of a reasonable expectation of success can be implicit,” for example “where the Board makes an implicit finding on reasonable expectation of success by considering and addressing other, intertwined arguments, including, as we hold today, a motivation to combine.” Because the Board’s express motivation to combine findings and related, implicit reasonable expectation of success findings were supported by substantial evidence, the Federal Circuit affirmed its decision that the challenged claims were unpatentable as obvious.
The opinion can be found here.
By Jason A. Wrubleski
This article summarizes aspects of the law and does not constitute legal advice. For legal advice for your situation, you should contact an attorney.
Sign up
